¶ Updated on 6 Aug 2021 by Frank Lee.
Background: BosLab has strains of colorful E. coli for use in agar art classes and competitions. These strains are the standard lab strain DH5α with a plasmid inserted that contains a colorful protein gene plus an antibiotic resistance gene against chloramphenicol. When grown in culture media containing chloramphenicol, bacterial cells containing the plasmid should have a fitness advantage, providing an incentive for cells to retain copies of the plasmid as they divide and propagate.
Making the colorful protein, however, is an additional metabolic burden on the cells. BosLab members Wendy and Kellen noted that, over several re-culturings and storage in our 4°C refrigerator over the years, more and more cells seem to lose their color, yet continue to propagate in the chloramphenicol-laced media. You can see this in the form of colorless colonies on the streak plates shown in Figure 1; we’d expect all colonies to be colored, but this isn’t so. This suggests that there are cells which have disabling mutations in the color gene but retain a functioning chloramphenicol resistance gene. To keep the cultures colorfast, we may periodically need to hand select for colorful cells and re-propagate.
Starting with the colorful E. coli on these LB agar + chloramphenicol antibiotic plates:
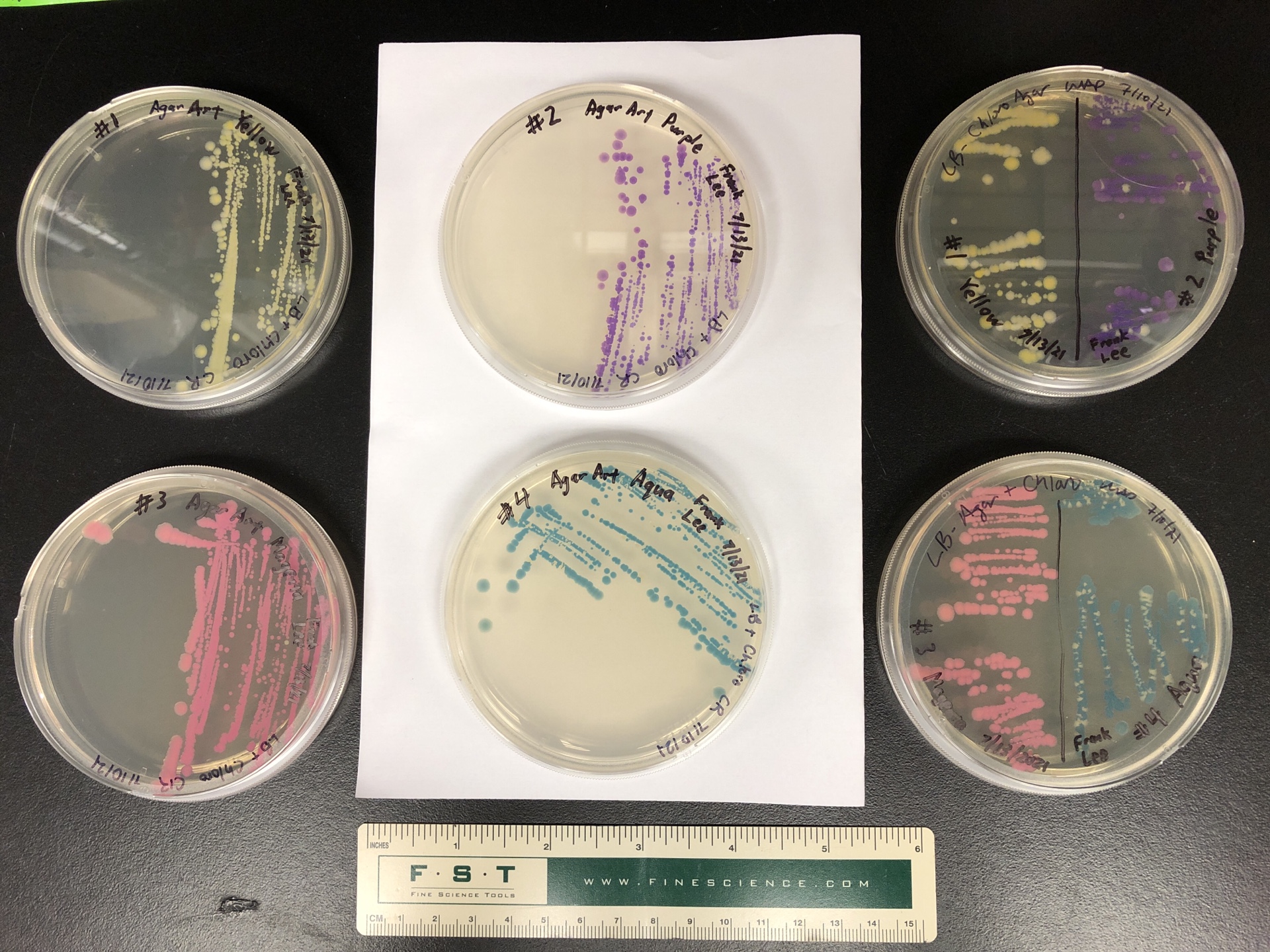
Goal: To select and maintain colorful E. coli strains that can be re-cultured indefinitely while remaining colorfast.
Method summary: I'll choose the most robust-looking colonies for each color and grow them in liquid LB+chloramphenicol, then streak the resulting cultures onto plates and repeat (i.e. choose colonies from the new plates, grow in fresh liquid LB+chloramphenicol, streak onto plates to get new colonies to choose from) again and again until all streaked plates grow 100% colored colonies at least five times in a row. This protocol is not guaranteed to succeed by any means, but I'm willing to take a chance and spend some plates, culture tubes, media, time, and effort on this.
Secondary goal: To create opportunities to practice sterile culturing techniques regularly, to provide a gentle introduction to lab technique for members who are new to biology lab work, all while serving the lab community by maintaining these strains.
¶ Detailed protocol (newest first):
- Oct 5, 2023 revision of Liquid Culturing
- Dec 3, 2021 revision of Liquid Culturing
- Sept 14, 2021 revision of Liquid Culturing
- Aug 1, 2021 revision of Liquid Culturing
- July 20, 2021 revision of Liquid Culturing.
¶ Log entries, oldest to newest:
- 2021 July 18: Liquid cultures all coloring nicely except Aqua tube #2. Added a new Aqua tube #3 from a freshly picked colony from the streak plates.
- 2021 July 20: Made streak plates from the liquid cultures. Had to make new plates using Wendy's protocol (or 7/20/2021 protocol snapshot).
- 2021 July 21: Checked plates: Growth noted but with many uncolored colonies.
- 2021 July 23: Picked colonies from plates to inoculate liquid cultures.
- 2021 July 25: Control tube reveals contamination in the LB media. The contaminant doesn't appear to grow with chloramphenicol antibiotic in the media.
- 2021 July 30: Streaked plates.
- 2021 August 1: All colonies on the plates were colored as expected.
- 2021 December 3: Started liquid cultures of 6 colors, two culture tubes per color. The colors are Yellow, Lime, Teal, Pink, Blue, Purple. (protocol snapshot)
- 2021 December 4: The liquid cultures all turned cloudy except three of the four control tubes, which we expected, and the two Yellow tubes, which we did not anticipate. We will need to try again, though perhaps not until after the 10 December agar art class. The plate that I got the Yellow culture from dates from 30 July, which may be too long ago for most of the cells to remain viable.
¶ 1 Aug 2021
Came in to look at the streak plates.
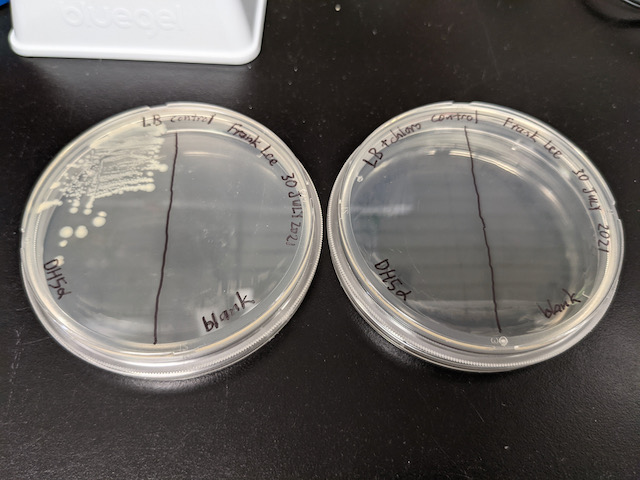
The controls (Figure 11b below) behaved as expected per our predictions in Table 1 on July 30, 2021. That is, the only sector that showed any visible growth was “LB+DH5α”. This suggests that the LB agar is free from contamination and the chloramphenicol antibiotic is effective.
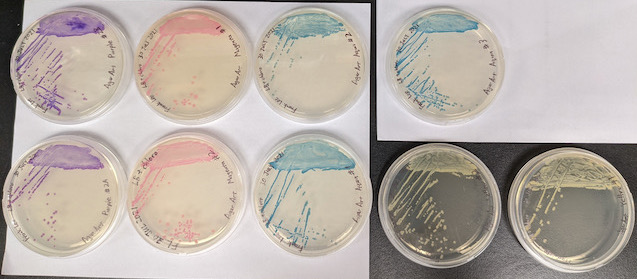
The purple, magenta, and aqua plates have 100% colored colonies:
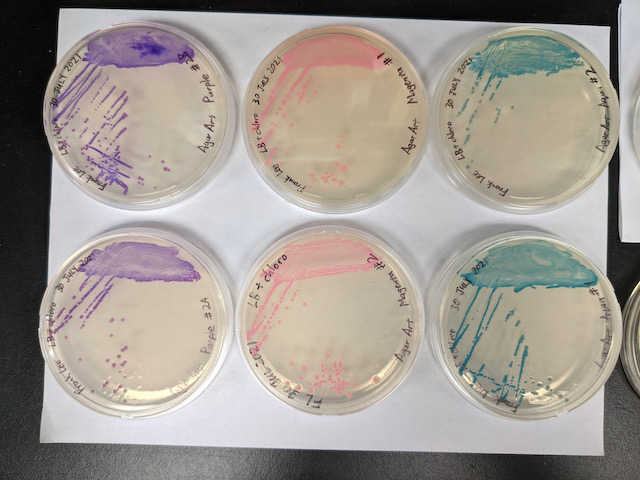
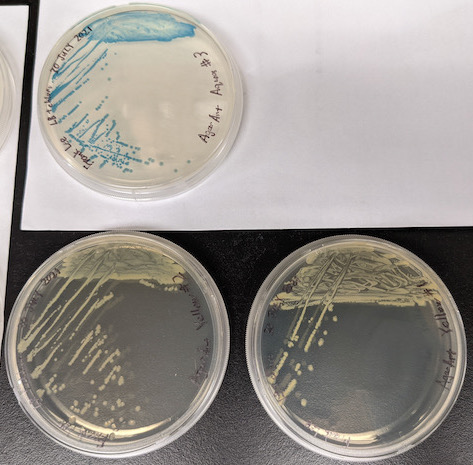
I stored the plates in the 4⁰C lab fridge to slow down growth until we have an opportunity to pick colonies to inoculate liquid cultures.
¶ 4 Aug 2021
Fellow BosLab member Chris Watt and I incubated liquid cultures from the streak plates today. Chris had two additional colors of bacteria to share, one I'll call Neon Green, and the other simply Blue. We followed the protocol with N=6 to reflect that we now have six colors total. Left the tubes incubating in the orbital shaker at 36°C and 240 rpm. The 36°C is my mistake in programming the incubator. I intended 37°C.
[TBD: insert photo of new color plates]
¶ 5 Aug 2021
Came to check on the culture tubes. No substantial color yet.
[TBD: insert photo of agar art strain tubes]
The control tubes suggest that the inoculation was without contamination, that the LB is good, and the antibiotic is effective.
[TBD: insert photo of control tubes]
¶ 6 Aug 2021
Came in to pour some LB only plates for DH5α culture maintenance and some LB+chloro plates for streaking the agar art cultures tomorrow. I now think it is best to let plates fully dry and solidify overnight so the agar is hardened and isn't so easily scratched by the inoculation loop during streaking.
Oops… there's a possibility that I put in only half the amount of antibiotic needed into the agar before pouring the plates. It'll be interesting to see the “LB+chloro+DH5α” control plate when we make one tomorrow.
EOF