¶ Revised 2023 Oct 5+6 by Frank Lee
Changes from 3 Dec 2021 revision: New kanamycin-resistant agar art strains:
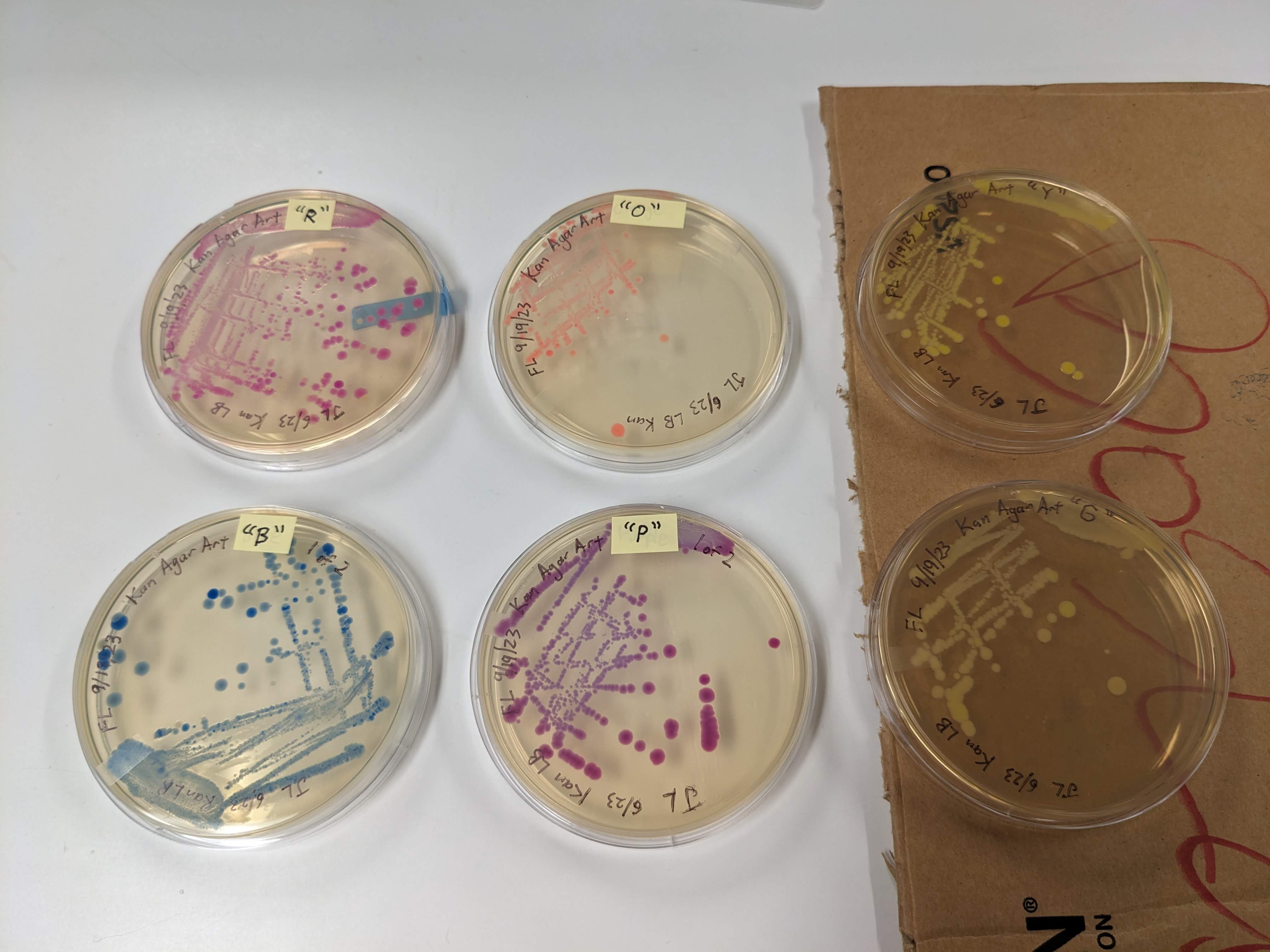
Colors we have today:
- “R”: looks like a dark magenta
- “O”: looks like a slightly yellowish pink
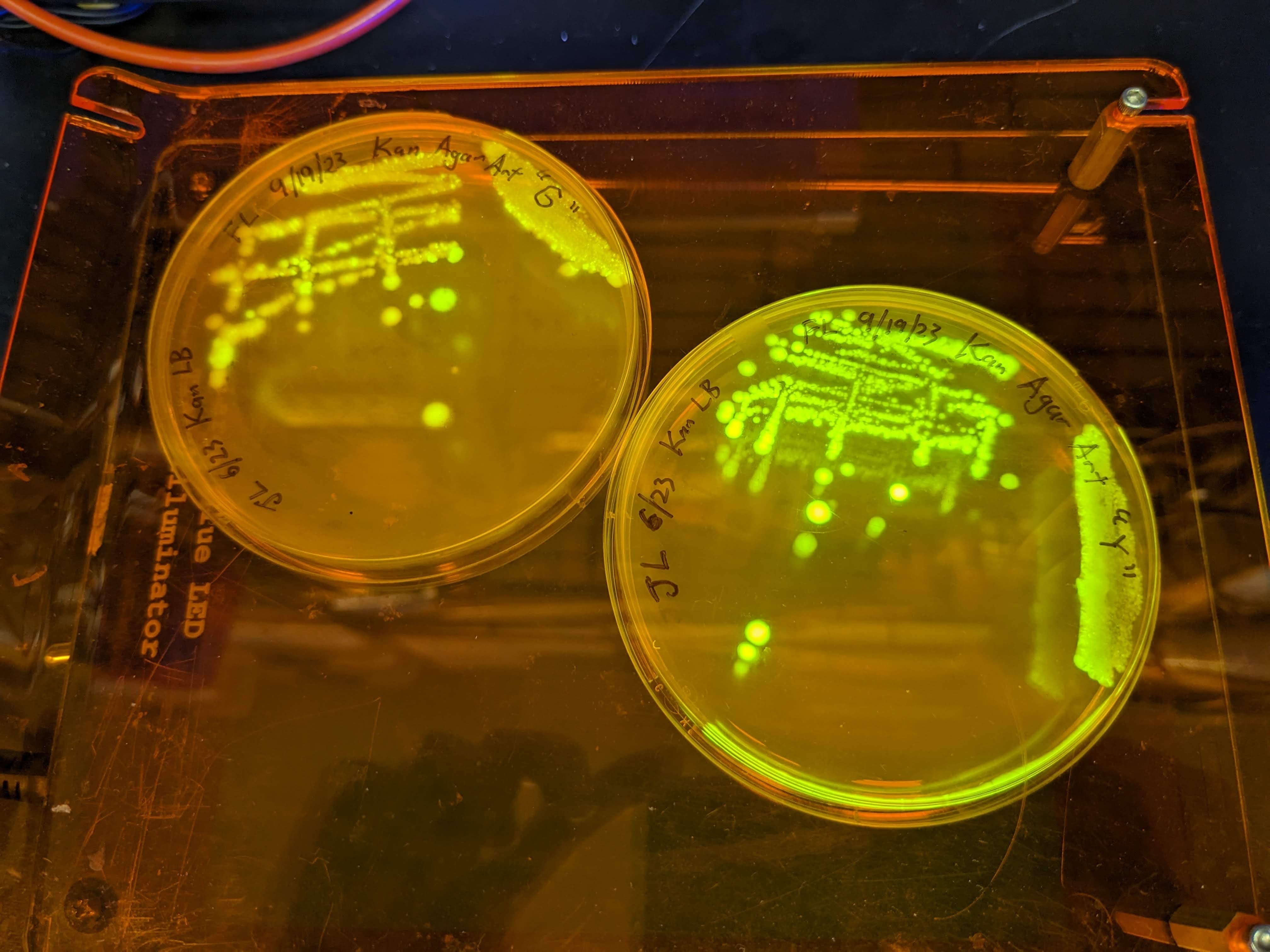
- “Y”: looks like a greenish yellowish white; most colonies glow bright neon green under UV light
- “G”: looks like a version of “Y” with mostly off-white colonies and some greenish yellowish white colonies; a few colonies glow neon green under UV light but most do not glow very noticeably
- “B”: looks dark blue with the tiniest hint of green; some colonies are a lighter bluish gray
- “P”: looks like a reddish dark purple, a bit like a more bluish version of “R”
“Y” and “G” do not appear to be very distinct colors in office lighting.

Materials and equipment needed for N strains: (N=6 for us)
| For N=6 different strains: | |
| 2 × N sterile 5 mL culture tubes with caps | 16 culture tubes = 12 culture tubes + 4 control tubes |
|
4 sterile 5 mL culture tubes, with caps, as controls:
|
|
| Rack/holder for above culture tubes | I used 2 racks with 16 open spaces each so I could start with all the tubes on one rack. Then as I perform a protocol step on a tube, I can move it to the other rack to indicate that that tube has completed the current step. This helps me track my progress in case I get distracted while performing the protocol (e.g. a lab partner needs help). |
| Marker pen for labeling tubes | Use a VWR Lab Marker. Avoid Sharpie markers as their ink is NOT alcohol-fast, and in our lab we use alcohol to sanitize surfaces and our gloved hands often, so it is very easy to smudge labels written with Sharpies. The VWR Lab Markers use an ink that is alcohol-resistant once dry. |
| Stock bottle of at least (2N+4+2) x 1 mL of sterile liquid LB media |
Liquid LB amount needed: 2N+4+2 = 2x6 + 4 + 2 = 18 We'll use 20 mL, which is 18 mL plus 2 mL extra just in case
|
| 1 sterile serological pipette to aliquot (2N+4+2) x 1 mL | could use a 25 mL sterile disposable pipette to transfer the 20 mL LB aliquot. We used a 10 mL pipette three times to deliver 10 mL + 10 mL + 5 µL = 25 mL. |
| sterile culture media bottle to hold the LB media aliquot mixed with kanamycin | an empty, clean, autoclaved 100 mL glass media bottle |
| pipettor for serological pipettes (e.g. pipette-aid) | should check that the pipettor doesn't leak and has good batteries |
| a pipette to transfer 1 mL (i.e. 1000 µL) | will use a P1000 set to 1000 µL |
| 1000X kanamycin stock solution, 50 mg/mL, from the −20°C freezer |
1000x kanamycin stock solution needed: 20 µL (i.e. use 1 µL stock solution per mL of media)
|
| micropipettor to deliver N × 10 µL |
use a P20 (i.e. 20 µL capacity pipettor)
|
| 1 sterile micropipette tip for the above |
get the box of P20 tips
|
| (2 × N + 4) sterile inoculation loops all from the same bag or container | 16 sterile inoculation loops |
| Plates with streaked, colorful, agar art colonies from the fridge or incubator | the 6 single-color agar plates marked “Kan Agar Art” |
| Laminar flow hood | reserve the BosLab PCR Workstation |
Protocol: (quantities are for N=6 strains)
- Turn on the laminar flow hood. Wipe down the work surface with ≥70% ethanol. Let dry.
- Bring materials inside the laminar flow hood. The following steps MUST be done in the laminar air flow to ensure sterility.
- Open the bag of sterile culture tubes inside laminar flow and take out the tubes needed without reaching into the bag, thus avoid contaminating the remaining tubes in the bag. Close bag when done.
- Line up all tubes on the first row of the rack.
- Label two tubes for each color. For example, “Y1”, “Y2”, “P1”, “P2”. Also write your name, date, and the words “LB+kan”.
- Label four control tubes as follows
- “LB only control”
- “LB + DH5α control”
- “LB + kan only control”
- “LB + kan + DH5α control”.
- Open a sterile bottle of liquid LB.
- Open the 100 mL bottle, that is, the aliquot-receiving container.
- Aliquot the LB: Use the serological pipette to add 25 mL of sterile LB to the 100 mL bottle.
- Today, 6 Oct 2023, we added 25 mL instead of 20, as noted in the Materials section.
- Close the stock LB bottle immediately to prevent inadvertent contamination.
- Use the P1000 to transfer 1 mL of the LB aliquot to these two control tubes:
- "LB only control"
- “LB + DH5α control”
- Add antibiotic to the LB aliquot: Use the P20 micropipette to transfer 20 µL of kanamycin stock solution to the LB aliquot bottle.
- Today we used the P200 to transfer 50 µL of kanamycin stock to produce 2x concentrated media for incubation.
- 1000x kanamycin is frozen solid when it comes out of the -20ºC, so I thawed it in my hands, taking about 5 minutes to do so. I noticed that the thawed solution has white powder at the bottom and some white chunks floating at the top, so I vortexed the tube for about a minute to resuspend before using.
- Gently swirl the aliquoted bottle to mix in the kanamycin.
- Use the P1000 to transfer 1 mL of the LB+kanamycin mix to each empty tube including any empty control tubes. It is OK to use the same pipette tip as you used to fill the “LB only control” tube if and only if you're certain you haven't contaminated the pipette by touching the tip to any surface.
- For each color, choose the most strongly colored colonies that don't have other colonies touching them. Prefer colonies that look circular. Circle the two chosen colonies on each plate using a marker on the back of the plate.
- Using a new sterile inoculation loop:
- Pick up the chosen colony.
- Swirl into the correspondingly labeled sterile culture tube. You may be able to see the colored agar plate scraping on the inoculation loop come off the loop into the LB + kan liquid in the tube, providing positive reassurance that cells from the colony were indeed transferred.
- Dispose of the used loop in the red biohazard waste baggie in the laminar flow.
- Repeat for the 2nd tube of that color.
- Repeat the above two steps until you have 2 inoculated tubes for each color.
- For the controls today, I opened a tube from the -20°C from a freezer box labeled “DH5α competent cells 200 µL JL”. These appeared to be glycerol stocks, and the cells seemed to all be clustered in a pellet at the bottom of the tube. I vortexed for about 15 seconds to resuspend the cells before using.
- Incubate the tubes in the orbital shake table incubator overnight at 37°C and 250 rpm.
- The cultures are ready to be streaked out onto plates the next day, for testing. (See Wendy's protocol for streaking plates.) Hopefully you get 100% colored colonies.
- Don't incubate too long before streaking. Some strains seem to lose the ability to express color well if you incubate more than a day or two before streaking plates.
- Repeat this procedure until you get at least 5 times in a row where all streaked colonies are colored for all colors. Then perhaps we can say that we've succeeded in rescuing robust, colorfast strains for each color!
Parent project: Maintaining Agar Art strains at BosLab.
I performed this protocol on 6 Oct 2023.
